Cefmenoxime is a third-generation cephalosporin antibiotic.[citation needed]
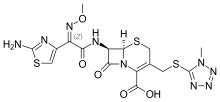 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Intramuscular, intravenous |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 100% (given IM) |
| Protein binding | 50% to 70% |
| Metabolism | Negligible |
| Elimination half-life | 1 hour |
| Excretion | Kidney, unchanged |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H17N9O5S3 |
| Molar mass | 511.55 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Synthesis
editThe alkylation of ethyl 2-hydroxyimino-3-oxobutanoate (1) with dimethylsulfate gives ethyl (2Z)-2-methoxyimino-3-oxo-butanoate (2). Halogenation with molecular bromine leads to ethyl 4-bromo-2-methoxyimino-3-oxobutanoate (3). Treatment with thiourea gives ethyl (Z)-2-(2-amino-4-thiazolyl)-2-methoxyiminoacetate (4) which is reacted with chloroacetyl chloride to give the amide (5). Saponification with potassium hydroxide gives (6) which is halogenated with phosphorus pentachloride to (7). Amide formation with the cephalosporin intermediate (8) then gives (9). Removal of the protecting group with benzyltriethylammonium bromide yields (10). The tert-butyl ester was deprotected with trifluoroacetic acid to give (11). Lastly, thioether formation with 5-mercapto-1-methyltetrazole (12) completes the synthesis of cefmenoxime.[1][2][3][4]
References
edit- ^ Michihiko Ochiai, et al. U.S. patent 4,098,888 (1978 to Takeda Pharmaceutical Co Ltd).
- ^ Ochiai, Michihiko; Aki, Osami; Morimoto, Akira; et al. (1977). "New cephalosporin derivatives with high antibacterial activities". Chemical and Pharmaceutical Bulletin. 25 (11): 3115–3117. doi:10.1248/cpb.25.3115.
- ^ Ochiai, Michihiko; Morimoto, Akira; Miyawaki, Toshio; et al. (1981). "Synthesis and structure-activity relationships of 7 beta-(2-(2-aminothiazol-4-yl)acetamido)cephalosporin derivatives. V. Synthesis and antibacterial activity of 7 beta-(2-(2-aminothiazol-4-yl)-2-methoxyiminoacetamido)cephalosporin derivatives and related compounds". The Journal of Antibiotics. 34 (2): 171–185. doi:10.7164/antibiotics.34.171. PMID 6271716.
- ^ Ochiai, Michihiko; Morimoto, Akira; Miyawaki, Toshio (1981). "Synthesis and structure-activity relationships of 7 beta-(2-(2-aminothiazol-4-yl)acetamido)cephalosporin derivatives. VI. Alternative syntheses of 7 beta-(2-(2-aminothiazol-4-yl)-(Z)-2-methoxyiminoacetamido)cephalosporin derivatives". The Journal of Antibiotics. 34 (2): 186–192. doi:10.7164/antibiotics.34.186. PMID 6271717.
External links
edit- Diseases Database (DDB): 30892
- Yokota N, Koguchi M, Suzuki Y, Fukayama S, Ishihara R, Deguchi K, Oda S, Tanaka S, Nakane Y, Fukumoto T (1995). "Antibacterial activities of cefmenoxime against recent fresh clinical isolates from patients in sinusitis". Jpn J Antibiot. 48 (5): 602–9. PMID 7637194.
- Paladino J, Fell R (1994). "Pharmacoeconomic analysis of cefmenoxime dual individualization in the treatment of nosocomial pneumonia". Ann Pharmacother. 28 (3): 384–9. doi:10.1177/106002809402800316. PMID 8193431. S2CID 29444681.
- Duncker G, Reich U, Krausse R (1994). "Cefmenoxime in corneal organ culture". Ophthalmologica. 208 (5): 262–6. doi:10.1159/000310505. PMID 7816419.