Monoaminergic activity enhancers (MAE), also known as catecholaminergic/serotonergic activity enhancers (CAE/SAE), are a class of compounds that enhance the action potential-evoked release of monoamine neurotransmitters in the nervous system.[1] MAEs are distinct from monoamine releasing agents (MRAs) like amphetamine and fenfluramine in that they do not induce the release of monoamines from synaptic vesicles but rather potentiate only nerve impulse propagation-mediated monoamine release.[2][3] That is, MAEs increase the amounts of monoamine neurotransmitters released by neurons per electrical impulse.[2][3]
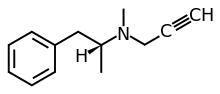
MAEs have been shown to significantly enhance nerve impulse-mediated dopamine release in the striatum, substantia nigra, and olfactory tubercle; norepinephrine release from the locus coeruleus; and/or serotonin release from the raphe nucleus in rodent studies.[4] Some MAEs are selective for effects on some of these neurotransmitters but not on others.[2][4] The maximal impacts of MAEs on brain monoamine levels are much smaller than with monoamine releasing agents like amphetamine and monoamine reuptake inhibitors like methylphenidate.[4][5] MAEs have a peculiar and characteristic bimodal concentration–response relationship, with two bell-shaped curves of MAE activity across tested concentration ranges.[2][6][4][7][8] Hence, there is a narrow concentration range for optimal pharmacodynamic activity.[6]
Endogenous MAEs include certain trace amines like phenylethylamine and tryptamine, while synthetic MAEs include certain phenethylamine and tryptamine derivatives like selegiline and benzofuranylpropylaminopentane (BPAP).[2] The actions of MAEs may be mediated by agonism of the trace amine-associated receptor 1 (TAAR1).[2][9][10][7] Antagonists of MAEs, like the TAAR1 antagonist EPPTB and rasagiline, have been identified.[10][7]
Endogenous monoaminergic activity enhancers
editA few endogenous MAEs have been identified, including β-phenylethylamine (PEA), tryptamine, and tyramine.[2][11] These MAEs possess selectivity for enhancing the release of a certain monoamine neurotransmitter over others.[2][4] For example, β-phenylethylamine is roughly 100 times more selective for potentiating dopamine release over serotonin release.[12] In contrast to β-phenylethylamine, tryptamine appears to selectively potentiate serotonin release over dopamine release.[2] The monoamine-potentiating effects of β-phenylethylamine and tyramine are distinct from their monoamine-releasing properties, which are only present at much higher concentrations.[13][11]
Exogenous MAEs like β-phenylethylamine, tryptamine, and tyramine are ineffective as MAEs in vivo in animals due to very rapid breakdown by monoamine oxidase.[2][11] However, monoamine oxidase inhibitors (MAOIs), specifically MAO-B inhibitors like selegiline, can dramatically potentiate β-phenylethylamine by inhibiting its metabolism and thereby allow for it to produce significant pharmacodynamic effects.[14][15][16][17] In addition, tyramine, unlike β-phenylethylamine and tryptamine, is unable to cross the blood–brain barrier, which limits its capacity for centrally mediated effects.[2][18]
Monoaminergic activity enhancing drugs
editSelegiline (L-deprenyl) (a phenylethylamine derivative) is used as an antiparkinsonian agent and antidepressant and exhibits CAE effects independent of its monoamine oxidase inhibition.[13] It has been shown to enhance both impulse-evoked norepinephrine and dopamine release.[7] Selegiline shows a bimodal concentration–response relationship in terms of its CAE actions for dopamine activity in the striatum.[7] Besides enhancing catecholaminergic activity, it has additionally been found to decrease serotonergic activity.[19] Aside from selegiline, D-deprenyl is also a CAE, with slightly lower potency than selegiline.[19] By extension to selegiline and D-deprenyl, the racemic form, deprenyl, is a CAE.[19] A halogenated analogue of deprenyl, 4-fluorodeprenyl, has been found to act as a CAE as well.[19]
The psychostimulants amphetamine (both levoamphetamine and dextroamphetamine) and methamphetamine (both levomethamphetamine and dextromethamphetamine) are CAEs like selegiline, but these drugs are also potent monoamine releasing agents and these actions overshadow the former activities.[1][20][10][19] Levomethamphetamine, levoamphetamine, and dextroamphetamine are all similarly potent as CAEs and compared to selegiline, but are substantially more potent as CAEs than dextromethamphetamine.[19] Besides acting as CAEs, levomethamphetamine and dextromethamphetamine diminish serotonergic activity, similarly to selegiline, whereas levoamphetamine and dextroamphetamine do not do so.[19]
Phenylpropylaminopentane (PPAP) is a CAE for norepinephrine and dopamine that was derived from selegiline.[21] In contrast to selegiline, it lacks monoamine oxidase inhibition and hence is much more selective in its actions.[22]
Indolylpropylaminopentane (IPAP) is a MAE for serotonin, norepinephrine, and dopamine that was derived from tryptamine.[4][10][23] It shows some selectivity for serotonin, with its maximal impact on this neurotransmitter occurring at 10-fold lower concentrations than for norepinephrine or dopamine.[10][23]
Benzofuranylpropylaminopentane (BPAP) is a MAE for serotonin, norepinephrine, and dopamine that was derived from tryptamine.[2][24] It is about 130 times more potent in its MAE actions than selegiline.[2] Similarly to selegiline, BPAP shows a bimodal concentration–response relationship in its MAE effects, specifically on norepinephrine activity in the locus coeruleus.[2][6][8]
In contrast to selegiline, rasagiline and its metabolite (R)-1-aminoindan do not have MAE actions.[7][25] Similarly, SU-11739 (AGN-1133; J-508), the N-methylated analogue of rasagiline and a closer analogue of selegiline, does not have MAE actions.[25]
Mechanism of action
editThe mechanism of action of MAEs, for instance the trace amines, may be explained by their shared affinities for the trace amine-associated receptor 1 (TAAR1).[2][9] In addition, recent findings have suggested that known synthetic MAEs like BPAP and selegiline may exert their effects via TAAR1 activation.[10][7] This was evidenced by the TAAR1 antagonist EPPTB reversing their MAE effects, among other findings.[10][7]
MAEs require transport into monoaminergic neurons by monoamine transporters (MATs) like the dopamine transporter (DAT).[10] Hence, they must be substrates of these transporters in order to exert their MAE effects.[10] This may be due to the fact that the TAAR1 is located intracellularly within neurons.[10] Transport by MATs into monoaminergic neurons is similarly required for the releasing effects of monoamine releasing agents like amphetamine.[10] The TAAR1 is also thought to be involved in the releasing effects of monoamine releasing agents as with MAEs.[10] It has been proposed that there may be two distinct binding sites on the TAAR1, one for MAEs and one for monoamine releasing agents.[10] MAEs are thought to induce action potential-dependent vesicular monoamine release via TAAR1 activation, whereas monoamine releasing agents are thought to induce impulse-independent non-vesicular monoamine release via TAAR1 activation.[10]
As with monoamine releasing agents like amphetamine and monoamine reuptake inhibitors like methylphenidate, single acute doses of MAEs rapidly increase brain monoamine levels.[4][5] However, MAEs have more limited impacts on brain monoamine levels compared to monoamine releasing agents and monoamine reuptake inhibitors.[4] In an in vivo rodent study, BPAP was found to maximally increase dopamine levels in the striatum by 44%, in the substantia nigra by 118%, and in the olfactory tubercle by 57%; norepinephrine levels in the locus coeruleus by 228%; and serotonin levels in the raphe nucleus by 166%.[4][26] The maximal impacts of other MAEs like selegiline on brain monoamine levels are similar.[4] For comparison, the norepinephrine–dopamine releasing agent amphetamine increases dopamine levels in the striatum by 700 to 1,500% of baseline and norepinephrine levels in the prefrontal cortex by 400 to 450% of baseline.[27][27] However, there appears to be no dose–effect ceiling with this agent and it can maximally increase striatal dopamine levels by more than 5,000% of baseline at higher doses.[27][5][28] Monoamine reuptake inhibitors including methylphenidate, atomoxetine, bupropion, and vanoxerine (GBR-12909) also robustly increase brain monoamine levels in rodents, though the maximal impacts of these agents are much smaller (e.g., 5- to 10-fold lower) than those of releasers like amphetamine.[27][5]
MAEs like PPAP and BPAP have been found to increase locomotor activity, increase stereotyped behavior, facilitate learning and retention, and produce antidepressant-like effects in rodent studies.[21][29] In relation to these effects, they have been described as having psychostimulant-like effects.[21][29] The locomotor stimulant effect of BPAP has been shown to be dependent on enhancement of dopaminergic signaling.[29] In contrast to PPAP and BPAP, as well as in contrast to amphetamines, selegiline does not stimulate locomotor activity and lacks psychostimulant-like effects in rodents.[30] Accordingly, selegiline does not appear to activate the mesolimbic dopamine pathway in rodents.[31][16]
Antagonists
editAntagonists of MAEs are known.[4] For example, 3-F-BPAP, a derivative of BPAP, antagonizes the MAE actions of BPAP.[4] However, it does not antagonize the MAE actions of selegiline or PPAP.[4] EPPTB, a TAAR1 antagonist, has been found to reverse the MAE actions of both BPAP and selegiline.[10][7] Likewise, rasagiline has been found to reverse the MAE actions of selegiline and has been proposed as a possible TAAR1 antagonist.[7]
Enhancer regulation system and age-related changes
editAn endogenous enhancer regulation system for monoaminergic neurons has been proposed to exist in which so-called enhancer substances can potentiate the action potential-evoked release of monoamine neurotransmitters in a variety of brain areas.[6][4] This has also been referred to as the "mesencephalic enhancer regulation" system to emphasize the key importance of dopaminergic neurons and their modulation of behavior in this system.[6][4] However, enhancer-sensitive neurons are also present outside of the mesencephalon (midbrain) and activity enhancers can affect noradrenergic and serotonergic neurons as well.[4][6] Enhancer effects have even been observed in the peripheral nervous system.[2] The enhancer regulation system has been theorized to play an important role in dynamically controlling innate and acquired drives and mediating age-related changes in goal-directed behavioral activity.[6][4] The concept of this system was created and advanced by the developers of selegiline, including József Knoll and Ildikó Miklya.[13] Endogenous enhancer substances like phenethylamine and tryptamine are known, but are of relatively low potency.[6][4] The key endogenous actors in the enhancer regulation system have been hypothesized to be much more potent and have yet to be identified.[6][4]
Rodents are much more behaviorally and motivationally active in the late developmental phase of life (2 months) than in the early post-developmental phase (4 months).[4][13][32] This has been specifically quantified with orienting-searching reflex activity induced by hunger.[4][32] Male rats are weaned at about 3 weeks of age and complete sexual development by 2 months of age.[4][32] Subsequent research found that brain monoamine release is much higher during the developmental phase (4 weeks of age) compared to prior to weaning (2 weeks of age) or following sexual maturity (16–32 weeks of age).[4][13][32] This has included dopamine release in the striatum, substantia nigra, and olfactory tubercle; norepinephrine release in the locus coeruleus; and serotonin release in the raphe nucleus.[4][13][32] Serotonin release was 6- to 7-fold higher at 4 weeks of age compared to 2 weeks of age, whereas dopamine and norepinephrine release in their respective areas was around 2-fold higher relative to pre-weaning and post-sexual maturity.[4][32] In addition, monoamine release progressively declines with age going from 4 weeks to 32 weeks.[32] The higher behavioral activity of rodents at 2 months of age compared to before or after this age has been attributed to greater activity of the brain catecholaminergic system at this time.[4][13][32]
As previously described, brain monoamine release begins to rapidly decrease with sexual maturity in rodents.[4][13] This suggests that sex hormones and the onset of their production may dampen brain monoamine release.[4][13] Accordingly, brain monoamine release was found to be significantly higher in prepubertally castrated rats at 3 months of age compared to non-castrated controls.[4][33] In addition, treatment of 3-week-old prepubertal rats for 2 weeks with exogenous sex hormones, including the androgen testosterone or the estrogen estrone, though not progesterone, significantly and rapidly reduced brain monoamine release relative to untreated controls.[4][13][33] Similarly, sexual activity following sexual maturity substantially declines with age in both male rodents and humans.[4] This is thought to be due to age-related decreased activity of the brain dopaminergic system.[4]
It is known that brain levels of phenethylamine, a known endogenous enhancer substance, decline with age.[4] This may be due to progressively increased levels of MAO-B with age.[4] Decreased levels of phenethylamine may contribute to reduced activation of the enhancer regulation system and reduced brain catecholamine release with age.[6] However, the key endogenous actors of the enhancer regulation system are thought to be more potent than phenethylamine and have yet to be identified.[6][4] It has been hypothesized that highly potent enhancer substances may exist that may be able to rapidly modulate the activity of brain catecholaminergic neurons by as much as 5- to 10-fold to quickly control time-dependent motivational states.[34][11] However, such mediators remain speculative and have not been discovered as of present.[34][11][13][2][4]
Rodent studies have found that exogenous MAEs like selegiline and BPAP augment brain monoamine release, slow monoaminergic neurodegeneration, and help to preserve behavioral activity with age.[4][13][20][32] As an example, selegiline has been found to augment and delay loss of sexual performance in rodents.[4][35] It has been proposed that exogenous MAEs like selegiline might be able to modestly slow the age-related decay of brain monoamine release in humans, although such hypotheses have yet to be tested.[6][34][13][36][37]
Medical use
editSelegiline is currently the only MAE without concomitant potent monoamine releasing agent actions that is available for medical use.[4] It is also a selective MAO-B inhibitor and is used in the treatment of Parkinson's disease and depression.[4][13] According to József Knoll, one of the original developers of selegiline, the CAE effects of selegiline may be more important than MAO-B inhibition in terms of its effectiveness for Parkinson's disease.[4] This is consistent with clinical findings that selegiline may be more effective in the treatment of Parkinson's disease than rasagiline.[13][7][38]
Selective MAEs have been proposed for potential medical use in the treatment of a variety of conditions.[2][6][39][40][21] These include psychiatric disorders like depression and attention deficit hyperactivity disorder (ADHD) as well as neurodegenerative diseases like Parkinson's disease and Alzheimer's disease.[2][6][39][40][21] There has also been theoretical interest in MAEs as potential antiaging agents that might help to oppose age-related catecholaminergic neurodegeneration and prolong lifespan, though such ideas have not been tested.[13] BPAP in particular has been proposed for potential clinical development.[2][1][41] However, no other MAEs besides selegiline have been developed for medical use as of present.[1][4][13]
List of monoamine activity enhancers
editReferences
edit- ^ a b c d e f Knoll J (2001). "Antiaging compounds: (-)deprenyl (selegeline) and (-)1-(benzofuran-2-yl)-2-propylaminopentane, [(-)BPAP], a selective highly potent enhancer of the impulse propagation mediated release of catecholamine and serotonin in the brain". CNS Drug Rev. 7 (3): 317–45. doi:10.1111/j.1527-3458.2001.tb00202.x. PMC 6494119. PMID 11607046.
- ^ a b c d e f g h i j k l m n o p q r s t u v Shimazu, Seiichiro; Miklya, Ildikó (2004-05-01). "Pharmacological studies with endogenous enhancer substances: β-phenylethylamine, tryptamine, and their synthetic derivatives". Progress in Neuro-Psychopharmacology and Biological Psychiatry. 28 (3): 421–427. doi:10.1016/j.pnpbp.2003.11.016. ISSN 0278-5846. PMID 15093948. S2CID 37564231.
- ^ a b Bhattacharjee, Monojit; Perumal, Ekambaram (2019-03-01). "Potential plant-derived catecholaminergic activity enhancers for neuropharmacological approaches: A review". Phytomedicine. 55: 148–164. doi:10.1016/j.phymed.2018.07.010. ISSN 0944-7113. PMID 30668425. S2CID 58948967.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq Knoll J (2005). "Enhancer Regulation: A Neurochemical Approach to the Innate and Acquired Drives". The Brain and Its Self: A Neurochemical Concept of the Innate and Acquired Drives. Berlin/Heidelberg: Springer-Verlag. p. 25–94. doi:10.1007/3-540-27434-0_4. ISBN 978-3-540-23969-7.
- ^ a b c d Heal DJ, Smith SL, Kulkarni RS, Rowley HL (August 2008). "New perspectives from microdialysis studies in freely-moving, spontaneously hypertensive rats on the pharmacology of drugs for the treatment of ADHD". Pharmacol Biochem Behav. 90 (2): 184–197. doi:10.1016/j.pbb.2008.03.016. PMID 18456311.
- ^ a b c d e f g h i j k l m n Knoll J (August 2003). "Enhancer regulation/endogenous and synthetic enhancer compounds: a neurochemical concept of the innate and acquired drives". Neurochem Res. 28 (8): 1275–1297. doi:10.1023/a:1024224311289. PMID 12834268.
- ^ a b c d e f g h i j k Harsing LG, Timar J, Miklya I (August 2023). "Striking Neurochemical and Behavioral Differences in the Mode of Action of Selegiline and Rasagiline". Int J Mol Sci. 24 (17): 13334. doi:10.3390/ijms241713334. PMC 10487936. PMID 37686140.
- ^ a b Knoll J, Miklya I, Knoll B, Yasusa T, Shimazu S, Yoneda F (September 2002). "1-(Benzofuran-2-yl)-2-(3,3,3-trifluoropropyl)aminopentane HCl, 3-F-BPAP, antagonizes the enhancer effect of (-)-BPAP in the shuttle box and leaves the effect of (-)-deprenyl unchanged". Life Sci. 71 (17): 1975–84. doi:10.1016/s0024-3205(02)01968-9. PMID 12175892.
- ^ a b Berry MD (January 2007). "The potential of trace amines and their receptors for treating neurological and psychiatric diseases". Rev Recent Clin Trials. 2 (1): 3–19. doi:10.2174/157488707779318107. PMID 18473983.
In addition, the compounds previously described by Knoll and colleagues [33, 34], along with a series of trace amine derivatives synthesized by Ling et al. [35] are potential TAAR ligands. Although neither of these classes of compound appear to have been examined for efficacy at TAAR, their strong structural similarity to trace amines suggests that such studies are warranted.
- ^ a b c d e f g h i j k l m n o p Harsing LG, Knoll J, Miklya I (August 2022). "Enhancer Regulation of Dopaminergic Neurochemical Transmission in the Striatum". Int J Mol Sci. 23 (15): 8543. doi:10.3390/ijms23158543. PMC 9369307. PMID 35955676.
- ^ a b c d e Knoll J, Miklya I, Knoll B, Markó R, Rácz D (1996). "Phenylethylamine and tyramine are mixed-acting sympathomimetic amines in the brain". Life Sci. 58 (23): 2101–2114. doi:10.1016/0024-3205(96)00204-4. PMID 8649195.
- ^ Nakamura, M.; Ishii, A.; Nakahara, D. (1998-05-22). "Characterization of beta-phenylethylamine-induced monoamine release in rat nucleus accumbens: a microdialysis study". European Journal of Pharmacology. 349 (2–3): 163–169. doi:10.1016/s0014-2999(98)00191-5. ISSN 0014-2999. PMID 9671094.
- ^ a b c d e f g h i j k l m n o p q Miklya, I. (November 2016). "The significance of selegiline/(-)-deprenyl after 50 years in research and therapy (1965-2015)". Molecular Psychiatry. 21 (11): 1499–1503. doi:10.1038/mp.2016.127. ISSN 1476-5578. PMID 27480491. S2CID 205202709.
- ^ Yasar S, Goldberg JP, Goldberg SR (January 1, 1996). "Are metabolites of l-deprenyl (Selegiline) useful or harmful? Indications from preclinical research". Deprenyl — Past and Future. Journal of Neural Transmission. Supplementum. Vol. 48. pp. 61–73. doi:10.1007/978-3-7091-7494-4_6. ISBN 978-3-211-82891-5. PMID 8988462.
- ^ Timár J, Knoll B (January 1986). "The effect of repeated administration of (-) deprenyl on the phenylethylamine-induced stereotypy in rats". Arch Int Pharmacodyn Ther. 279 (1): 50–60. PMID 3083795.
- ^ a b Timár J, Gyarmati Z, Tekes K, Härsing GL, Knoll J (November 1993). "Further proof that (-)deprenyl fails to facilitate mesolimbic dopaminergic activity". Pharmacol Biochem Behav. 46 (3): 709–714. doi:10.1016/0091-3057(93)90566-c. PMID 8278449.
- ^ McKean AJ, Leung JG, Dare FY, Sola CL, Schak KM (2015). "The Perils of Illegitimate Online Pharmacies: Substance-Induced Panic Attacks and Mood Instability Associated With Selegiline and Phenylethylamine". Psychosomatics. 56 (5): 583–587. doi:10.1016/j.psym.2015.05.003. PMID 26198572.
- ^ Gillman PK (November 2018). "A reassessment of the safety profile of monoamine oxidase inhibitors: elucidating tired old tyramine myths". J Neural Transm (Vienna). 125 (11): 1707–1717. doi:10.1007/s00702-018-1932-y. PMID 30255284.
- ^ a b c d e f g h Knoll J, Miklya I (1994). "Multiple, small dose administration of (-)deprenyl enhances catecholaminergic activity and diminishes serotoninergic activity in the brain and these effects are unrelated to MAO-B inhibition". Arch Int Pharmacodyn Ther. 328 (1): 1–15. PMID 7893186.
- ^ a b Knoll J (February 1998). "(-)Deprenyl (selegiline), a catecholaminergic activity enhancer (CAE) substance acting in the brain". Pharmacol Toxicol. 82 (2): 57–66. doi:10.1111/j.1600-0773.1998.tb01399.x. PMID 9498233.
- ^ a b c d e f g Knoll, J.; Knoll, B.; Török, Z.; Timár, J.; Yasar, S. (1992). "The pharmacology of 1-phenyl-2-propylamino-pentane (PPAP), a deprenyl-derived new spectrum psychostimulant". Archives Internationales de Pharmacodynamie et de Therapie. 316: 5–29. ISSN 0003-9780. PMID 1356324.
- ^ Csaba G, Kovács P, Pállinger E (January–February 2006). "Acute and delayed effect of (-) deprenyl and (-) 1-phenyl-2-propylaminopentane (PPAP) on the serotonin content of peritoneal cells (white blood cells and mast cells)". Cell Biochemistry and Function. 24 (1): 49–53. doi:10.1002/cbf.1183. PMID 15584092. S2CID 11027835.
- ^ a b c Yoneda F, Moto T, Sakae M, Ohde H, Knoll B, Miklya I, Knoll J (May 2001). "Structure-activity studies leading to (-)1-(benzofuran-2-yl)-2-propylaminopentane, ((-)BPAP), a highly potent, selective enhancer of the impulse propagation mediated release of catecholamines and serotonin in the brain". Bioorg Med Chem. 9 (5): 1197–212. doi:10.1016/s0968-0896(01)00002-5. PMID 11377178.
- ^ Magyar, Kálmán; Lengyel, Joseph; Bolehovszky, Andrea; Knoll, Bertha; Miklya, Iidikó; Knoll, Joseph (2002-09-01). "The fate of (−)1-(benzofuran-2-yl)-2-propylaminopentane · HCl, (−)-BPAP, in rats, a potent enhancer of the impulse-evoked release of catecholamines and serotonin in the brain". European Journal of Drug Metabolism and Pharmacokinetics. 27 (3): 157–161. doi:10.1007/BF03190451. ISSN 2107-0180. PMID 12365195. S2CID 30618267.
- ^ a b Miklya I (June 2014). "Essential difference between the pharmacological spectrum of (-)-deprenyl and rasagiline". Pharmacol Rep. 66 (3): 453–458. doi:10.1016/j.pharep.2013.11.003. PMID 24905523.
- ^ Knoll J, Yoneda F, Knoll B, Ohde H, Miklya I (December 1999). "(-)1-(Benzofuran-2-yl)-2-propylaminopentane, [(-)BPAP], a selective enhancer of the impulse propagation mediated release of catecholamines and serotonin in the brain". Br J Pharmacol. 128 (8): 1723–1732. doi:10.1038/sj.bjp.0702995. PMC 1571822. PMID 10588928.
- ^ a b c d Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present--a pharmacological and clinical perspective". J Psychopharmacol. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
- ^ Cheetham SC, Kulkarni RS, Rowley HL, Heal DJ (2007). The SH rat model of ADHD has profoundly different catecholaminergic responses to amphetamine's enantiomers compared with Sprague-Dawleys. Neuroscience 2007, San Diego, CA, Nov 3-7, 2007. Society for Neuroscience. Archived from the original on 27 July 2024.
Both d- and l-[amphetamine (AMP)] evoked rapid increases in extraneuronal concentrations of [noradrenaline (NA)] and [dopamine (DA)] that reached a maximum 30 or 60 min after administration. However, the [spontaneously hypertensive rats (SHRs)] were much more responsive to AMP's enantiomers than the [Sprague-Dawleys (SDs)]. Thus, 3 mg/kg d-AMP produced a peak increase in [prefrontal cortex (PFC)] NA of 649 ± 87% (p<0.001) in SHRs compared with 198 ± 39% (p<0.05) in SDs; the corresponding figures for [striatal (STR)] DA were 4898 ± 1912% (p<0.001) versus 1606 ± 391% (p<0.001). At 9 mg/kg, l-AMP maximally increased NA efflux by 1069 ± 105% (p<0.001) in SHRs compared with 157 ± 24% (p<0.01) in SDs; the DA figures were 3294 ± 691% (p<0.001) versus 459 ± 107% (p<0.001).
- ^ a b c Shimazu S, Takahata K, Katsuki H, Tsunekawa H, Tanigawa A, Yoneda F, Knoll J, Akaike A (June 2001). "(-)-1-(Benzofuran-2-yl)-2-propylaminopentane enhances locomotor activity in rats due to its ability to induce dopamine release". Eur J Pharmacol. 421 (3): 181–189. doi:10.1016/s0014-2999(01)01040-8. PMID 11516435.
- ^ Timár J, Gyarmati Z, Barna L, Knoll B (August 1996). "Differences in some behavioural effects of deprenyl and amphetamine enantiomers in rats". Physiol Behav. 60 (2): 581–587. doi:10.1016/s0031-9384(96)80035-7. PMID 8840922.
- ^ Gyarmati S, Hársing LG, Tekes K, Knoll J (1990). "Repeated administration of (-)deprenyl leaves the mesolimbic dopaminergic activity unchanged". Acta Physiol Hung. 75 Suppl: 133–134. PMID 2115226.
- ^ a b c d e f g h i Knoll J, Miklya I (1995). "Enhanced catecholaminergic and serotoninergic activity in rat brain from weaning to sexual maturity: rationale for prophylactic (-)deprenyl (selegiline) medication". Life Sci. 56 (8): 611–620. doi:10.1016/0024-3205(94)00494-d. PMID 7869839.
- ^ a b Knoll J, Miklya I, Knoll B, Dalló J (July 2000). "Sexual hormones terminate in the rat: the significantly enhanced catecholaminergic/serotoninergic tone in the brain characteristic to the post-weaning period". Life Sci. 67 (7): 765–773. doi:10.1016/s0024-3205(00)00671-8. PMID 10968406.
- ^ a b c Knoll J (August 1994). "Memories of my 45 years in research". Pharmacol Toxicol. 75 (2): 65–72. doi:10.1111/j.1600-0773.1994.tb00326.x. PMID 7971740.
- ^ Knoll J, Yen TT, Miklya I (1994). "Sexually low performing male rats die earlier than their high performing peers and (-)deprenyl treatment eliminates this difference". Life Sci. 54 (15): 1047–1057. doi:10.1016/0024-3205(94)00415-3. PMID 8152326.
- ^ Knoll, J. (2012). How Selegiline ((-)-Deprenyl) Slows Brain Aging. Bentham Science Publishers. ISBN 978-1-60805-470-1. Retrieved 28 July 2024.
- ^ Knoll, J. (2005). The Brain and Its Self: A Neurochemical Concept of the Innate and Acquired Drives. SpringerLink: Springer e-Books. Springer Berlin Heidelberg. ISBN 978-3-540-27434-6. Retrieved 28 July 2024.
- ^ Binde CD, Tvete IF, Gåsemyr J, Natvig B, Klemp M (September 2018). "A multiple treatment comparison meta-analysis of monoamine oxidase type B inhibitors for Parkinson's disease". Br J Clin Pharmacol. 84 (9): 1917–1927. doi:10.1111/bcp.13651. PMC 6089809. PMID 29847694.
- ^ a b Gaszner P, Miklya I (December 2004). "The use of the synthetic enhancer substances (-)-deprenyl and (-)-BPAP in major depression". Neuropsychopharmacol Hung. 6 (4): 210–220. PMID 15825677.
- ^ a b Gaszner P, Miklya I (January 2006). "Major depression and the synthetic enhancer substances, (-)-deprenyl and R-(-)-1-(benzofuran-2-yl)-2-propylaminopentane". Prog Neuropsychopharmacol Biol Psychiatry. 30 (1): 5–14. doi:10.1016/j.pnpbp.2005.06.004. PMID 16023777.
- ^ Magyar K, Lengyel J, Bolehovszky A, Knoll B, Miklya I, Knoll J (2002). "The fate of (-)1-(benzofuran-2-yl)-2-propylaminopentane . HCl, (-)-BPAP, in rats, a potent enhancer of the impulse-evoked release of catecholamines and serotonin in the brain". Eur J Drug Metab Pharmacokinet. 27 (3): 157–161. doi:10.1007/BF03190451. PMID 12365195.