This article needs additional citations for verification. (October 2010) |
A bioreactor is any manufactured device or system that supports a biologically active environment.[1] In one case, a bioreactor is a vessel in which a chemical process is carried out which involves organisms or biochemically active substances derived from such organisms. This process can either be aerobic or anaerobic. These bioreactors are commonly cylindrical, ranging in size from litres to cubic metres, and are often made of stainless steel.[citation needed] It may also refer to a device or system designed to grow cells or tissues in the context of cell culture.[2] These devices are being developed for use in tissue engineering or biochemical/bioprocess engineering.[citation needed]
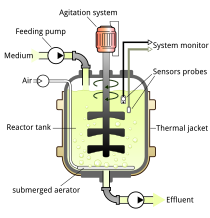
On the basis of mode of operation, a bioreactor may be classified as batch, fed batch or continuous (e.g. a continuous stirred-tank reactor model). An example of a continuous bioreactor is the chemostat.[citation needed]
Organisms or biochemically active substances growing in bioreactors may be submerged in liquid medium or may be anchored to the surface of a solid medium. Submerged cultures may be suspended or immobilized. Suspension bioreactors may support a wider variety of organisms, since special attachment surfaces are not needed, and can operate at a much larger scale than immobilized cultures. However, in a continuously operated process the organisms will be removed from the reactor with the effluent. Immobilization is a general term describing a wide variety of methods for cell or particle attachment or entrapment.[3] It can be applied to basically all types of biocatalysis including enzymes, cellular organelles, animal and plant cells and organs.[4][5] Immobilization is useful for continuously operated processes, since the organisms will not be removed with the reactor effluent, but is limited in scale because the microbes are only present on the surfaces of the vessel.
Large scale immobilized cell bioreactors are:
- moving media, also known as moving bed biofilm reactor (MBBR)
- packed bed
- fibrous bed
- membrane
Design
editBioreactor design is a relatively complex engineering task, which is studied in the discipline of biochemical/bioprocess engineering. Under optimum conditions, the microorganisms or cells are able to perform their desired function with limited production of impurities. The environmental conditions inside the bioreactor, such as temperature, nutrient concentrations, pH, and dissolved gases (especially oxygen for aerobic fermentations) affect the growth and productivity of the organisms. The temperature of the fermentation medium is maintained by a cooling jacket, coils, or both. Particularly exothermic fermentations may require the use of external heat exchangers. Nutrients may be continuously added to the fermenter, as in a fed-batch system, or may be charged into the reactor at the beginning of fermentation. The pH of the medium is measured and adjusted with small amounts of acid or base, depending upon the fermentation. For aerobic (and some anaerobic) fermentations, reactant gases (especially oxygen) must be added to the fermentation. Since oxygen is relatively insoluble in water (the basis of nearly all fermentation media), air (or purified oxygen) must be added continuously. The action of the rising bubbles helps mix the fermentation medium and also "strips" out waste gases, such as carbon dioxide. In practice, bioreactors are often pressurized; this increases the solubility of oxygen in water. In an aerobic process, optimal oxygen transfer is sometimes the rate limiting step. Oxygen is poorly soluble in water—even less in warm fermentation broths—and is relatively scarce in air (20.95%). Oxygen transfer is usually helped by agitation, which is also needed to mix nutrients and to keep the fermentation homogeneous. Gas dispersing agitators are used to break up air bubbles and circulate them throughout the vessel.[citation needed]
Fouling can harm the overall efficiency of the bioreactor, especially the heat exchangers. To avoid it, the bioreactor must be easily cleaned. Interior surfaces are typically made of stainless steel for easy cleaning and sanitation. Typically bioreactors are cleaned between batches, or are designed to reduce fouling as much as possible when operated continuously. Heat transfer is an important part of bioreactor design; small vessels can be cooled with a cooling jacket, but larger vessels may require coils or an external heat exchanger.[citation needed]
Types
editPhotobioreactor
editA photobioreactor (PBR) is a bioreactor which incorporates some type of light source (that may be natural sunlight or artificial illumination). Virtually any translucent container could be called a PBR, however the term is more commonly used to define a closed system, as opposed to an open storage tank or pond. Photobioreactors are used to grow small phototrophic organisms such as cyanobacteria, algae, or moss plants.[6] These organisms use light through photosynthesis as their energy source and do not require sugars or lipids as energy source. Consequently, risk of contamination with other organisms like bacteria or fungi is lower in photobioreactors when compared to bioreactors for heterotroph organisms.[citation needed]
Sewage treatment
editConventional sewage treatment utilises bioreactors to undertake the main purification processes. In some of these systems, a chemically inert medium with very high surface area is provided as a substrate for the growth of biological film. Separation of excess biological film takes place in settling tanks or cyclones. In other systems aerators supply oxygen to the sewage and biota to create activated sludge in which the biological component is freely mixed in the liquor in "flocs". In these processes, the liquid's biochemical oxygen demand (BOD) is reduced sufficiently to render the contaminated water fit for reuse. The biosolids can be collected for further processing, or dried and used as fertilizer. An extremely simple version of a sewage bioreactor is a septic tank whereby the sewage is left in situ, with or without additional media to house bacteria. In this instance, the biosludge itself is the primary host for the bacteria.[citation needed]
Bioreactors for specialized tissues
editMany cells and tissues, especially mammalian ones, must have a surface or other structural support in order to grow, and agitated environments are often destructive to these cell types and tissues. Higher organisms, being auxotrophic, also require highly specialized growth media. This poses a challenge when the goal is to culture larger quantities of cells for therapeutic production purposes, and a significantly different design is needed compared to industrial bioreactors used for growing protein expression systems such as yeast and bacteria.[citation needed]
Many research groups have developed novel bioreactors for growing specialized tissues and cells on a structural scaffold, in attempt to recreate organ-like tissue structures in-vitro. Among these include tissue bioreactors that can grow heart tissue,[7][8] skeletal muscle tissue,[9] ligaments, cancer tissue models, and others. Currently, scaling production of these specialized bioreactors for industrial use remains challenging and is an active area of research.
For more information on artificial tissue culture, see tissue engineering.
Modelling
editMathematical models act as an important tool in various bio-reactor applications including wastewater treatment. These models are useful for planning efficient process control strategies and predicting the future plant performance. Moreover, these models are beneficial in education and research areas.[citation needed]
Bioreactors are generally used in those industries which are concerned with food, beverages and pharmaceuticals. The emergence of biochemical engineering is of recent origin. Processing of biological materials using biological agents such as cells, enzymes or antibodies are the major pillars of biochemical engineering. Applications of biochemical engineering cover major fields of civilization such as agriculture, food and healthcare, resource recovery and fine chemicals.[citation needed]
Until now, the industries associated with biotechnology have lagged behind other industries in implementing control over the process and optimization strategies. A main drawback in biotechnological process control is the problem of measuring key physical and biochemical parameters.[10]
Operational stages in a bio-process
editA bioprocess is composed mainly of three stages—upstream processing, bioreaction, and downstream processing—to convert raw material to finished product.[11]
The raw material can be of biological or non-biological origin. It is first converted to a more suitable form for processing. This is done in an upstream processing step which involves chemical hydrolysis, preparation of liquid medium, separation of particulate, air purification and many other preparatory operations.[citation needed]
After the upstream processing step, the resulting feed is transferred to one or more bioreaction stages. The biochemical reactors or bioreactors form the base of the bioreaction step. This step mainly consists of three operations, namely, production of biomass, metabolite biosynthesis and biotransformation.[citation needed]
Finally, the material produced in the bioreactor must be further processed in the downstream section to convert it into a more useful form. The downstream process mainly consists of physical separation operations which include solid liquid separation, adsorption, liquid-liquid extraction, distillation, drying etc.[12]
Specifications
editA typical bioreactor consists of following parts:
Agitator – Used for the mixing of the contents of the reactor which keeps the cells in the perfect homogenous condition for better transport of nutrients and oxygen to the desired product(s).
Baffle – Used to break the vortex formation in the vessel, which is usually highly undesirable as it changes the center of gravity of the system and consumes additional power.
Sparger – In aerobic cultivation process, the purpose of the sparger is to supply adequate oxygen to the growing cells.
Jacket – The jacket provides the annular area for circulation of constant temperature of water which keeps the temperature of the bioreactor at a constant value.[13]
See also
edit- ATP test
- Biochemical engineering
- Biofuel from algae
- Biological hydrogen production (algae)
- Bioprocessor
- Bioreactor landfill
- Biotechnology
- Cell culture
- Chemostat
- Digester
- Electro-biochemical reactor (EBR)
- Hairy root culture
- History of biotechnology
- Hollow fiber bioreactor
- Immobilized enzyme
- Industrial biotechnology
- Moving bed biofilm reactor
- Septic tank
- Single-use bioreactor
- Tissue engineering
References
edit- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "bioreactor". doi:10.1351/goldbook.B00662
- ^ "Bioreactoes and Cultivation Systems for Cell and Tissue Culture" (PDF). eolss.net. Retrieved 12 August 2023.
- ^ López, Asunción; Lázaro, Nuria; Marqués, Ana M. (September 1997). "The interphase technique: a simple method of cell immobilization in gel-beads". Journal of Microbiological Methods. 30 (3): 231–234. doi:10.1016/S0167-7012(97)00071-7.
- ^ Kowalczyk, Tomasz; Sitarek, Przemysław; Toma, Monika; Rijo, Patricia; Domínguez‐Martín, Eva; Falcó, Irene; Sánchez, Gloria; Śliwiński, Tomasz (August 2021). "Enhanced Accumulation of Betulinic Acid in Transgenic Hairy Roots of Senna obtusifolia Growing in the Sprinkle Bioreactor and Evaluation of Their Biological Properties in Various Biological Models". Chemistry & Biodiversity. 18 (8): e2100455. doi:10.1002/cbdv.202100455. hdl:10261/247635. ISSN 1612-1872. PMID 34185351. S2CID 235672736.
- ^ Peinado, Rafael A.; Moreno, Juan J.; Villalba, Jose M.; González-Reyes, Jose A.; Ortega, Jose M.; Mauricio, Juan C. (December 2006). "Yeast biocapsules: A new immobilization method and their applications". Enzyme and Microbial Technology. 40 (1): 79–84. doi:10.1016/j.enzmictec.2005.10.040.
- ^ Decker, Eva L.; Reski, Ralf (14 August 2007). "Current achievements in the production of complex biopharmaceuticals with moss bioreactors". Bioprocess and Biosystems Engineering. 31 (1): 3–9. doi:10.1007/s00449-007-0151-y. PMID 17701058. S2CID 4673669.
- ^ Bursac, N.; Papadaki, M.; Cohen, R. J.; Schoen, F. J.; Eisenberg, S. R.; Carrier, R.; Vunjak-Novakovic, G.; Freed, L. E. (1 August 1999). "Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies". American Journal of Physiology. Heart and Circulatory Physiology. 277 (2): H433–H444. doi:10.1152/ajpheart.1999.277.2.h433. PMID 10444466.
- ^ Carrier, Rebecca L.; Papadaki, Maria; Rupnick, Maria; Schoen, Frederick J.; Bursac, Nenad; Langer, Robert; Freed, Lisa E.; Vunjak-Novakovic, Gordana (5 September 1999). "Cardiac tissue engineering: Cell seeding, cultivation parameters, and tissue construct characterization". Biotechnology and Bioengineering. 64 (5): 580–589. doi:10.1002/(SICI)1097-0290(19990905)64:5<580::AID-BIT8>3.0.CO;2-X. PMID 10404238.
- ^ Heher, Philipp; Maleiner, Babette; Prüller, Johanna; Teuschl, Andreas Herbert; Kollmitzer, Josef; Monforte, Xavier; Wolbank, Susanne; Redl, Heinz; Rünzler, Dominik; Fuchs, Christiane (September 2015). "A novel bioreactor for the generation of highly aligned 3D skeletal muscle-like constructs through orientation of fibrin via application of static strain". Acta Biomaterialia. 24: 251–265. doi:10.1016/j.actbio.2015.06.033. PMID 26141153.
- ^ Carlsson, Bengt (March 24, 2009). "An introduction to modeling of bioreactors" (PDF).
- ^ Rosser, J.; Thomas, D. J. (2018-01-01), Thomas, Daniel J.; Jessop, Zita M.; Whitaker, Iain S. (eds.), "10 - Bioreactor processes for maturation of 3D bioprinted tissue", 3D Bioprinting for Reconstructive Surgery, Woodhead Publishing, pp. 191–215, ISBN 978-0-08-101103-4, retrieved 2020-12-14
- ^ Jana, AMIYA K. (2011). CHEMICAL PROCESS MODELLING AND COMPUTER SIMULATION. PHI Learning Pvt. Ltd.[page needed]
- ^ "Bioreactor- Basics".
Further reading
edit- Pauline M Doran, Bio-process Engineering Principles, Elsevier, 2nd ed., 2013 ISBN 978-0-12-220851-5
- Biotechnology company