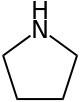
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like".[4] In addition to pyrrolidine itself, many substituted pyrrolidines are known.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Pyrrolidine[1] | |||
| Other names
Azolidine
Azacyclopentane Tetrahydropyrrole Prolamine Azolane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 102395 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.227 | ||
| EC Number |
| ||
| 1704 | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1922 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H9N | |||
| Molar mass | 71.123 g·mol−1 | ||
| Appearance | Clear colorless liquid | ||
| Density | 0.866 g/cm3 | ||
| Melting point | −63 °C (−81 °F; 210 K) | ||
| Boiling point | 87 °C (189 °F; 360 K) | ||
| Miscible | |||
| Acidity (pKa) | 11.27 (pKa of conjugate acid in water),[2] 19.56 (pKa of conjugate acid in acetonitrile)[3] | ||
| -54.8·10−6 cm3/mol | |||
Refractive index (nD)
|
1.4402 at 28°C | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
highly flammable, harmful, corrosive, possible mutagen | ||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H225, H302, H314, H332 | |||
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P301+P312, P301+P330+P331, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P330, P363, P370+P378, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 3 °C (37 °F; 276 K) | ||
| 345 °C (653 °F; 618 K) | |||
| Safety data sheet (SDS) | MSDS | ||
| Related compounds | |||
Related nitrogen heterocyclic compounds
|
Pyrrole (aromatic with two double bonds) Pyrroline (one double bond) Pyrrolizidine (two pentagonal rings) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Production and synthesis
editIndustrial production
editPyrrolidine is prepared industrially by the reaction of 1,4-butanediol and ammonia at a temperature of 165–200 °C and a pressure of 17–21 MPa in the presence of a cobalt- and nickel oxide catalyst, which is supported on alumina.[5]
The reaction is carried out in the liquid phase in a continuous tube- or tube bundle reactor, which is operated in the cycle gas method. The catalyst is arranged as a fixed-bed and the conversion is carried out in the downflow mode. The product is obtained after multistage purification and separation by extractive and azeotropic distillation.[5]
Laboratory synthesis
editIn the laboratory, pyrrolidine was usually synthesised by treating 4-chlorobutan-1-amine with a strong base:
Furthermore, 5-membered N-heterocyclic ring of the pyrrolidine derivatives can be synthesized via cascade reactions.[6]
Occurrence
editMany modifications of pyrrolidine are found in natural and synthetic drugs and drug candidates.[6] The pyrrolidine ring structure is present in numerous natural alkaloids i.a. nicotine and hygrine. It is found in many drugs such as procyclidine and bepridil. It also forms the basis for the racetam compounds (e.g. piracetam, aniracetam). The amino acids proline and hydroxyproline are, in a structural sense, derivatives of pyrrolidine.
Reactions
editPyrrolidine is a base. Its basicity is typical of other dialkyl amines.[7] Relative to many secondary amines, pyrrolidine is distinctive because of its compactness, a consequence of its cyclic structure.
Pyrrolidine is used as a building block in the synthesis of more complex organic compounds. It is used to activate ketones and aldehydes toward nucleophilic addition by formation of enamines (e.g. used in the Stork enamine alkylation):[8]
References
edit- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 142. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ Hall, H. K. (1957). "Correlation of the Base Strengths of Amines". Journal of the American Chemical Society. 79 (20): 5441–5444. doi:10.1021/ja01577a030.
- ^ Kaljurand, I.; Kütt, A.; Sooväli, L.; Rodima, T.; Mäemets, V.; Leito, I.; Koppel, I. A. (2005). "Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales". The Journal of Organic Chemistry. 70 (3): 1019–1028. doi:10.1021/jo048252w. PMID 15675863.
- ^ Pyrrolidine Archived 2017-11-21 at the Wayback Machine, The Good Scents Company
- ^ a b Bou Chedid, Roland; Melder, Johann-Peter; Dostalek, Roman; Pastre, Jörg; Tan, Aik Meam. "Process for the preparation of pyrrolidine". Google Patents. BASF SE. Archived from the original on 5 July 2019. Retrieved 5 July 2019.
- ^ a b Łowicki, Daniel; Przybylski, Piotr (2022). "Tandem construction of biological relevant aliphatic 5-membered N-heterocycles". European Journal of Medicinal Chemistry. 235: 114303. doi:10.1016/j.ejmech.2022.114303. PMID 35344904. S2CID 247580048.
- ^ H. K. Hall Jr. (1957). "Correlation of the Base Strengths of Amines". J. Am. Chem. Soc. 79 (20): 5441. doi:10.1021/ja01577a030.
- ^ R. B. Woodward, I. J. Pachter, and M. L. Scheinbaum (1974). "2,2-(Trimethylenedithio)cyclohexanone". Organic Syntheses. 54: 39
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 6, p. 1014.
External links
edit- Media related to Pyrrolidine at Wikimedia Commons